Exploiting the flexibility of the pyrochlore composition for acid-resilient iridium oxide electrocatalysts in proton exchange membranes†
Abstract
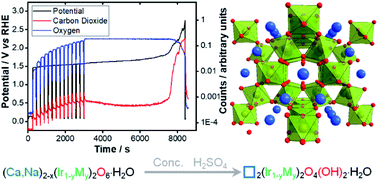
Iridate pyrochlore oxides (Na,Ca)2−x(Ir2−yMy)O6·nH2O (M = Sb, Zr, Ru, Rh) are studied as electrocatalysts for the oxygen evolution reaction under acid conditions. The materials crystallise from aqueous solution under alkali hydrothermal conditions with 10–40 nm crystallite size. Refinement of their crystal structures using both powder neutron and X-ray diffraction determined the composition of the materials, and Ir LIII-edge XANES spectroscopy shows the average Ir oxidation state to be close to 4.5 in all materials, consistent with bond valence sums. All materials show high electrocatalytic activity for the oxygen evolution reaction and the electrocatalyst which best maintains activity on cycling is the sodium-free Ca2−xIr2O6·nH2O, while the (Na,Ca)2−xIr2O6·nH2O material shows highest activity when normalised for surface area. In membrane electrode assemblies, carbon corrosion is minimised, making the materials suitable for use in catalyst layers in proton exchange membrane devices, such as electrolysers and fuel cells. Under strongly acidic conditions it is proved that while A-site Ca and Na are readily leached, the average pyrochlore structure is maintained, as is electrocatalytic activity, with charge balance achieved by inclusion of protons in the pyrochlore structure in the form of bridging hydroxyls, as seen using inelastic neutron scattering spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...