NiMoO4 nanorods with rich catalytic sites as a superb electrocatalyst for cerium-based flow batteries†
Abstract
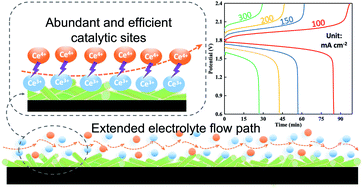
Higher cell voltage, current density and voltage efficiency are desired in redox flow batteries (RFBs) to enable the cells to achieve higher power density and energy efficiency. Cerium–vanadium flow batteries (Ce–V RFBs) have larger cell voltage than all-vanadium RFBs; however, the reaction kinetics of cerium ions is sluggish, limiting the current density and voltage efficiency. In this work, a novel binary metal oxide (NiMoO4) is uniformly deposited on a graphite felt electrode, which possesses a unique nanorod morphology with an extended pathway for electrolyte flow and forms an active site-rich surface enabling high electrocatalytic kinetics for the cerium redox reactions. To the best of our knowledge, NiMoO4 nanorods have not been reported in RFB research. The electrochemical performance of NiMoTGF is evaluated in a Ce–V flow cell, achieving 81.93% voltage efficiency at 150 mA cm−2, 26.7% higher than that of pristine graphite felt. Meanwhile, NiMoTGF enables the cell to charge at a high current density of 300 mA cm−2 and exhibits record voltage efficiency (70.03%). Additionally, the stability of NiMoTGF is demonstrated by 100 charge–discharge cycles showing good resistance toward corrosion caused by the oxidative cerium electrolyte. Furthermore, the catalytic mechanism of the NiMoO4 nanorod in cerium redox reactions is discussed.


 Please wait while we load your content...
Please wait while we load your content...