Pulsed electrocatalysis enables an efficient 2-electron oxygen reduction reaction for H2O2 production†
Abstract
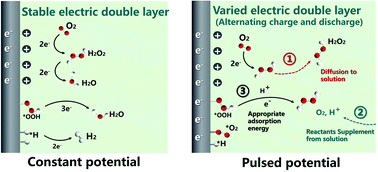
Hydrogen peroxide (H2O2) electrosynthesis through 2-electron oxygen reduction reaction (ORR) is an attractive alternative to the industrial anthraquinone oxidation method. Conventional H2O2 electrosynthesis processes usually operate under galvanostatic or potentiostatic conditions that occasionally result in unsatisfactory selectivity and efficiency. Herein, we applied pulsed electrocatalysis to achieve synergy between reactant adsorption, electrochemical reaction, and product diffusion by regulating the pulsed potential, pulsed width, and duty cycle, which could increase H2O2 production by 138.12%. For the first time, we revealed that pulsed electrocatalysis could regulate the adsorption of ORR intermediates by affecting the charge and discharge process of the electric double layer (EDL) based on density functional theory (DFT) calculations. Finally, we provided a new perspective on the mechanism of pulsed electrocatalysis to promote the production of H2O2via 2-electron ORR, thereby offering guidance for the precise control and efficient conduction of electrochemical reactions in the future.


 Please wait while we load your content...
Please wait while we load your content...