Simultaneous hydrogen and oxygen evolution reactions using free-standing nitrogen-doped-carbon–Co/CoOx nanofiber electrodes decorated with palladium nanoparticles†
Abstract
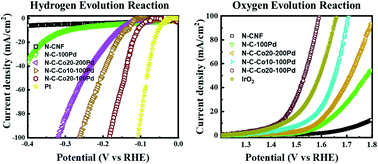
Designing efficient electrode materials for electrochemical water splitting is the most critical challenge for next-generation hydrogen fuel production. This study describes the development of free-standing nitrogen-doped carbon nanofiber (N-CNF) electrodes that incorporated cobalt/cobalt oxide (Co/CoOx) nanoparticles (NPs) and were decorated with palladium NPs (Pd NPs). These free-standing electrodes displayed high electrocatalytic activity during electrochemical water splitting, and were fabricated in three steps: (i) solution electrospinning of polyacrylonitrile (PAN)/cobalt acetate nanofiber mats; (ii) PAN/cobalt acetate nanofiber mat peroxidation and stabilization in air atmosphere followed by pyrolysis (carbonation) in nitrogen atmosphere; and (iii) decoration of the electrode surface with 5 and 10 nm Pd NPs by controlled atomic layer deposition (ALD) (100 and 200 cycles, respectively). The N-CNF–Co/CoOx–Pd electrode performance was tested in simultaneous hydrogen/oxygen evolution reactions (HER/OER) with an alkaline electrolyte solution (1 M KOH). The electrodes were as electroactive as Pt and IrO2 (reference electrodes) for overall electrochemical water splitting. The most efficient electrode displayed very interesting overpotential (100 mV and 160 mV @ j = 10 mA cm−2 for HER and OER, respectively), Tafel slope (33 and 113 mV dec−1), and exchange current density (1.15 and 22.4 mA cm−2) values. Interestingly, electrodes with the smallest Pd NP size (5 nm/100 ALD cycles) showed higher electrocatalytic activity for HER and OER than electrodes coated with bigger particles (10 nm/200 ALD cycles) and the reference Pt electrode. The effect of Co/CoOx NP encapsulation in the graphitic layers of N-CNFs and coating with Pd NPs on the electrode electrocatalytic activity are discussed in detail.
- This article is part of the themed collection: Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...