Electrochemical performance and reaction mechanism investigation of V2O5 positive electrode material for aqueous rechargeable zinc batteries†
Abstract
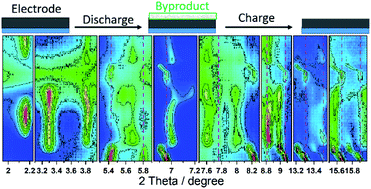
The electrochemical performance and reaction mechanism of orthorhombic V2O5 in 1 M ZnSO4 aqueous electrolyte are investigated. V2O5 nanowires exhibit an initial discharge and charge capacity of 277 and 432 mA h g−1, respectively, at a current density of 50 mA g−1. The material undergoes quick capacity fading during cycling under both low (50 mA g−1) and high (200 mA g−1) currents. V2O5 can deliver a higher discharge capacity at 200 mA g−1 than that at 50 mA g−1 after 10 cycles, which could be attributed to a different type of activation process under both current densities and distinct degrees of side reactions (parasitic reactions). Cyclic voltammetry shows several successive redox peaks during Zn ion insertion and deinsertion. In operando synchrotron diffraction reveals that V2O5 undergoes a solid solution and two-phase reaction during the 1st cycle, accompanied by the formation/decomposition of byproducts Zn3(OH)2V2O7·2(H2O) and ZnSO4Zn3(OH)6·5H2O. In the 2nd insertion process, V2O5 goes through the same two-phase reaction as that in the 1st cycle, with the formation of the byproduct ZnSO4Zn3(OH)6·5H2O. The reduction/oxidation of vanadium is confirmed by in operando X-ray absorption spectroscopy. Furthermore, Raman, TEM, and X-ray photoelectron spectroscopy (XPS) confirm the byproduct formation and the reversible Zn ion insertion/deinsertion in the V2O5.


 Please wait while we load your content...
Please wait while we load your content...