Electrochemically synthesized liquid-sulfur/sulfide composite materials for high-rate magnesium battery cathodes†
Abstract
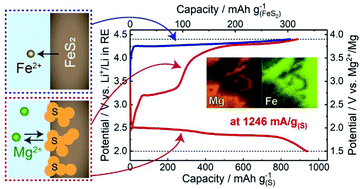
Mg/S batteries are some of the most promising rechargeable batteries owing to their high theoretical energy density. Their development is, however, hindered by (i) low electronic conductivity of S, (ii) sluggish Mg2+ diffusion in solid Mg–S compounds formed by discharge, and (iii) dissolubility of polysulfides into electrolytes. To address these problems, we propose liquid-S/sulfide composite cathode materials in combination with an ionic liquid electrolyte at intermediate temperatures (∼150 °C). The composite structure is spontaneously fabricated by electrochemically oxidizing metal sulfides, yielding liquid S embedded in a porous metal-sulfide conductive frame. This concept is demonstrated by a S/FeS2 composite cathode, which shows a significantly high-rate capability of, e.g., 1246 mA g−1(S) with a capacity of ∼900 mA h g−1(S). In addition, non-equilibrium liquid S formed by fast charging results in an unexpected higher discharge potential. This work provides a new strategy to design S-based cathodes for achieving high-rate multivalent rechargeable batteries.


 Please wait while we load your content...
Please wait while we load your content...