Carbonate formation lowers the electrocatalytic activity of perovskite oxides for water electrolysis†
Abstract
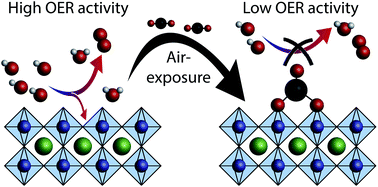
The study of oxide electrocatalysts is often complicated by the formation of complex and unknown surface species as well as the interaction between the catalysts and common support materials. Because unknown surface species may result from air exposure, we developed a clean transfer system for the air-free electrochemical investigation of epitaxial thin films fabricated under typical surface science conditions. LaNiO3 electrocatalysts exposed to ambient air exhibit a lower activity towards the oxygen evolution reaction than samples probed without air exposure. We demonstrate that this decrease in activity is connected to an alteration of the chemical environment of the electrocatalytically active sites through carbonate formation on exposure to CO2. Our study therefore shows that (1) the effects of air exposure must be considered for transition metal oxide catalysts and (2) that for the perovskite oxide LaNiO3 the clean surface is more active than the air-exposed surface.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...