Iron–oxygen covalency in perovskites to dominate syngas yield in chemical looping partial oxidation†
Abstract
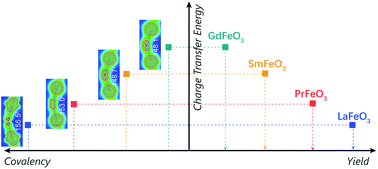
Perovskite-type (ABO3) oxygen carriers (OCs) with non-stoichiometric lattice oxygen release have been widely investigated in chemical looping partial oxidation processes (CLPO), and improving the syngas yield depends crucially on tailoring their elemental composition. Nevertheless, how their composition affects the electronic structure and finally the syngas yield remains elusive. Herein, by means of experiments and density functional theory calculations, we report that A-site lanthanide affects the Fe–O covalency in LnFeO3 (Ln = La, Pr, Sm, and Gd), controlling the syngas yield of OCs. Specifically, a small A-site cation radius induces severe geometric tilting of FeO6 octahedra, which weakens the Fe–O orbital hybridization in real space, and thus the Fe–O covalency in k space. The decrease in Fe–O covalency in k space can be gauged by the increasing charge-transfer energy, i.e., GdFeO3 > SmFeO3 > PrFeO3 > LaFeO3. The increase in the charge-transfer energy increases the formation energy of oxygen vacancies and the migration energy of oxygen anions, finally deteriorating oxygen mobility and surface oxygen activity. The activity and stability tests show that the syngas yields decreased in the order of LaFeO3 > PrFeO3 > SmFeO3 > GdFeO3, corresponding to the Fe–O covalency intensity. Our findings unlock a useful tool, i.e., charge-transfer energy, for rationally designing OCs and deepening our understanding of the modulating mechanism of lattice oxygen in chemical looping technologies.


 Please wait while we load your content...
Please wait while we load your content...