MOF-derived M-OOH with rich oxygen defects by in situ electro-oxidation reconstitution for a highly efficient oxygen evolution reaction†
Abstract
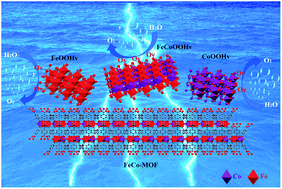
The oxygen evolution reaction (OER) is an important half-reaction in the field of energy production. However, effective, simple, and green preparation of low-cost OER electrocatalysts remains a problem. Herein, a novel in situ electro-oxidation reconstitution strategy is first adopted to derive metal-oxyhydroxides with rich oxygen-defects (M-OOHv) on the surface of Fe2Co-MOF with nickel foam (NF) as the substrate, named Fe2Co-MOF@M-OOHv-ER/NF, which exhibits an extremely low overpotential of 224 mV at a current density of 10 mA cm−2 in 1 M KOH for the OER, with a small Tafel slope of 44.87 mV dec−1 and a high electrochemically active surface area of 50.5 cm2. The in situ Raman reveals the transition of the MOF phase to the M-OOHv phase, which is the real active site for the OER. Furthermore, X-ray photoelectron spectroscopy, photoluminescence, and density functional theory calculations prove that the bimetal synergistic effect and the oxygen defects in the M-OOHv, regulating the electron density of states, are the real reasons for the higher catalytic activity for the OER. In short, the newly prepared Fe2Co-MOF@M-OOHv-ER/NF not only can be prepared by an efficient, simple, and green strategy, but also has excellent oxygen evolution performance.


 Please wait while we load your content...
Please wait while we load your content...