Phase evolution of electrochemically potassium intercalated graphite†
Abstract
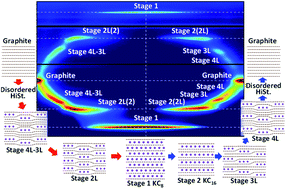
Graphite is commonly known as a layered material to accommodate alkali metal ions between graphene layers and is used as a negative electrode material of most Li-ion batteries and for developing K-ion batteries. Phase evolution of graphite during chemical K-vapor intercalation was well studied in the 1920s–1980s, but that during electrochemical (de)potassiation is different and not fully clarified yet. Herein, we elucidate the phase evolution behaviors of electrochemically potassium intercalated graphite by operando X-ray diffraction in comparison to those of a Li system based upon optimizing the graphite electrode and electrolyte conditions for precise analysis. The operando diffraction data and first-principles calculations results reveal staging transformations from graphite to stage-1 KC8 through disorderly stacked high stage, stage 4L, stage 3L, stage 2L, and stage 1 phases and reversal transitions including hysteresis. Based on the experimental and theoretical data, we propose structural change mechanisms of graphite during electrochemical K-intercalation and deintercalation on the basis of Daumas–Hérold defects and the defect disappearance by complete K-intercalation.


 Please wait while we load your content...
Please wait while we load your content...