Kinetics of the CO2 reduction reaction in aprotic Li–CO2 batteries: a model study
Abstract
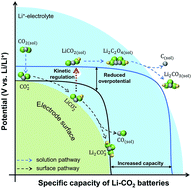
The aprotic Li–CO2 battery represents a promising technology that can potentially achieve energy conversion and storage and CO2 recycling simultaneously. However, current Li–CO2 batteries suffer from the sluggish kinetics of the CO2 reduction reaction (CO2RR) which often leads to high discharge overpotential, low energy efficiency, and low power densities. Toward unlocking the energy capabilities of Li–CO2 batteries, it is crucially important to have a fundamental understanding of the kinetics aspect of the Li–CO2 electrochemistry. Here we report a brief but comprehensive model to bridge the overall reaction kinetics and the elementary steps of the CO2RR in Li–CO2 batteries. A critical kinetics descriptor, i.e., the adsorption energy of the LiCO2 intermediate on the cathode surface, is proposed to reveal the interplay and competition between two different CO2RR mechanisms (i.e., the solution- and the surface-mediated pathways) occurring in Li–CO2 batteries. Our model indicates that tuning the CO2RR toward the solution-mediated pathway can avoid cathode surface passivation and is favorable for high-capacity and high-rate discharging of Li–CO2 batteries. The model study reported here sheds light on the kinetics aspect of Li–CO2 electrochemistry and would be beneficial for the design of better Li–CO2 batteries.


 Please wait while we load your content...
Please wait while we load your content...