Iron-doped titanium dioxide hollow nanospheres for efficient nitrogen fixation and Zn–N2 aqueous batteries†
Abstract
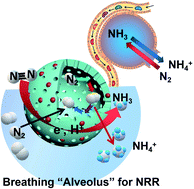
Electrochemical nitrogen reduction reactions (NRRs) show great potential for replacing the energy-intensive Haber–Bosch process, but require highly active electrocatalytic centers. Although various catalysts have been recently developed for NRRs, their inefficiency remained a huge obstacle for further commercialization. In this work, a shell-engineering strategy is proposed for the design of iron-doped titanium dioxide hollow nanospheres (FeHTNs) with abundant oxygen vacancies for NRRs based on the breathing process of mammalian alveolus. The hollow structure and porous shell of FeHTNs enable fast delivery of N2 reactants into the cavity of “alveolus” and stay in there longer and thus give N2 molecules more chance to contact the inner surface of electrocatalysts and hence greatly enhance NRR kinetics for the formation of ammonia. The dissolved ammonia in the electrolytic solution, in turn, creates negative pressure in cavities, which can suck N2 reactants automatically into the cavity, triggering the “domino effect” for continuous NRRs. A record ammonia yield of 43.14 μg h−1 mgcat.−1 at −0.7 V vs. RHE was achieved on Fe1.0HTN catalysts, close to the top of the pyramid among recently reported TO2-based catalysts. Besides, Fe1.0HTNs were employed as a N2 cathode for assembled Zn–N2 aqueous batteries, achieving both ammonia production (average NH3 yield: 0.172 μg h−1 cm−2) and electricity generation (power density: 16.42 μW cm−2), which offers a fascinating prospect for practical applications.


 Please wait while we load your content...
Please wait while we load your content...