Labile oxygen participant adsorbate evolving mechanism to enhance oxygen reduction in SmMn2O5 with double-coordinated crystal fields†
Abstract
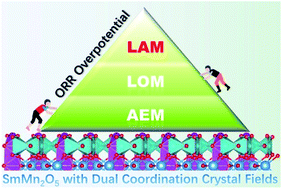
The current understanding of the oxygen reduction reaction (ORR) mechanism can fall into two categories: (1) the adsorbate evolving mechanism (AEM) over active metallic sites, in which all oxygen-containing intermediates originate from the electrolyte; (2) the lattice oxygen-mediated mechanism (LOM), in which the lattice oxygen in perovskite directly participates in the reaction. For more complicated metallic oxides with multiple ligand fields, these two mechanisms may fail to precisely describe the ORR process, as the local oxygen environment on the terminated surfaces of the catalyst is more variable relative to perovskites with only one type of ligand field. Herein, based on the constructed (SmMn2O5)n (n = 1, 2, 3, 4, 8) clusters and (001) slab model of a Mn-based mullite catalyst with a double-coordinated crystal field (Mn3+-centered square pyramid and octahedral crystal field centered on Mn4+), we discovered a new ORR mechanism, named the labile oxygen participant adsorbate evolving mechanism (LAM), via density functional theory calculations. Compared with the AEM, our proposed LAM further considers the labile oxygen participating in the reactions in the presence of intermediate OOH*, in contrast to the LOM, which does not involve OOH* formation. During the LAM, the formation of OOH* was determined to be the rate-limiting step. The moderate binding strength of the OOH* stems from the reasonable p–d orbital coupling between Mn–O bonds, trigged by the multiple oxygen coordination environments. The proposed LAM provides new insights into oxygen reactions over the more complicated catalysts with multiple ligands.


 Please wait while we load your content...
Please wait while we load your content...