Reduced grey brookite for noble metal free photocatalytic H2 evolution†
Abstract
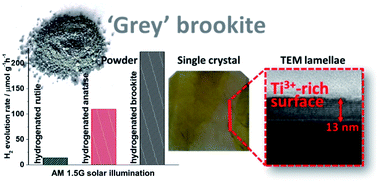
Herein we introduce for the first time a reduced “grey” brookite TiO2 photocatalyst, produced by thermal hydrogenation of brookite nanoparticles, that shows a remarkable noble metal free photocatalytic H2 evolution. Its activity is substantially higher than that of other TiO2 polymorphs, i.e. anatase or rutile, comparably sized and activated by hydrogenation under optimized conditions. Along with brookite powders, an oriented brookite single crystal was investigated as a defined surface to confirm the effects of the hydrogenation treatment. By a combination of electron paramagnetic resonance (EPR), electron and X-ray characterization techniques applied to the powders and single crystal, we find that hydrogenation forms in brookite a defective crystalline surface layer rich in Ti3+ states. Amorphization effects, i.e. forming the so called “amorphous shell” as reported in previous work for black anatase TiO2, were not detected. Overall, we provide experimental evidence that hydrogenation forms in brookite a surface strained zone with point defects that are mediators for electron transfer to H2O, leading to a significantly enhanced noble metal free photocatalytic H2 evolution in comparison to anatase.


 Please wait while we load your content...
Please wait while we load your content...