Phosphoric acid and thermal treatments reveal the peculiar role of surface oxygen anions in lithium and manganese-rich layered oxides†
Abstract
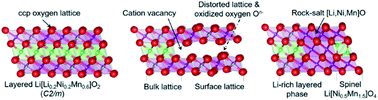
The interplay between cationic and anionic redox activity during electrochemical cycling makes layered Li-rich oxides appealing cathodes for state-of-the-art lithium-ion batteries. However, it remains challenging as the origin of lattice oxygen activity is not yet fully understood. Here we report on the effects of a lithium-deficient layer in the near-surface region of Co-free Li-rich Li[Li0.2Ni0.2Mn0.6]O2 (LLNMO) achieved via a phosphoric acid surface treatment. Our results show that oxidized On− (0 < n < 2) species are formed on the surface of H3PO4-treated LLNMO resulting from Li ion deficiency and lattice distortion. The metastable On− could be easily released from the oxygen surface lattice forming O2via thermal treatment, accompanied by a surface reconstruction and a layered-to-rock-salt/spinel transition. The presented results demonstrate that the surface lattice structure plays a critical role in the electrochemical performance of LLNMO. This information provides new insights into the oxygen redox in LLNMO and opens up an opportunity for Li-rich cathodes to achieve long cycle life toward a broad range of applications in electrical energy storage devices.


 Please wait while we load your content...
Please wait while we load your content...