Tuning the dynamic fragility of acrylic polymers by small molecules: the interplay of molecular structures†
Abstract
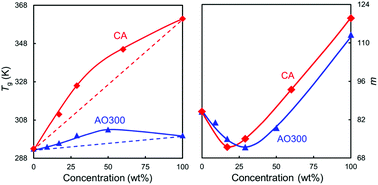
This report studied changes in the dynamic fragility (m) of poly(butyl methacrylate) (PBMA) by introducing guest hindered phenols capable of forming two or three intermolecular hydrogen bonds (inter-HBs) per molecule with the host polymer. The small molecules effectively decrease the m value, even if they apparently increase the glass transition temperature (Tg) of mixtures. The reduction in m was confirmed by enthalpy relaxation in two aspects: adding the guest molecule leads to a stronger cooling rate dependence of the limiting fictive temperature together with an apparent increase in aging rate of PBMA hybrids at low concentrations. By varying the molecule size and steric hindrance of the hydroxyl group on the hindered phenols, we clarified that m is primarily governed by the strength of inter-HB interactions, while the Tg value of mixtures depends on a combined effect of additive bulkiness and HB interaction. The anomalous dynamics was further rationalized not only by the HB-induced flexibility balance between side groups and backbone, but also by the reduction of cooperative rearranging sizes and alleviation of long-chain connectivity in such HB-driven hybrids.


 Please wait while we load your content...
Please wait while we load your content...