Electrochemical breakdown in hydrogel ionotronic devices
Abstract
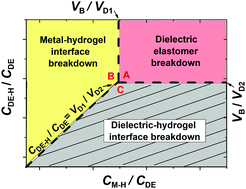
In recent years, hydrogel, as a stretchable, transparent, ionic conductor, has attracted considerable attention and its integration with various materials has enabled new functions: hydrogel ionotronics. These hybrid systems rely on both mobile ions and mobile electrons. However, coupling of ions and electrons brings a new challenge: electrochemical breakdown. Here, we study the breakdown behaviors of a typical ionotronic system—a hydrogel–elastomer device at high DC voltage, which consists of three elements: hydrogel, dielectric elastomer, and metal. We develop a phase diagram of the possible failure modes through theory and experiment, and find a new failure mode, electrochemical breakdown, caused by ion–electron exchange at the metal–hydrogel interface. Our experiments show that the breakdown voltage of the dielectric elastomer decreases when the capacitance of the electrical double layer formed at the metal–hydrogel interface is below a certain value. It is found that the failure mode and its transition are determined by three material properties: the electrical breakdown strength of the dielectric elastomer, the capacitance of the metal–hydrogel interface per unit area, and the electrochemical window of the hydrogel electrolyte. These findings will guide the characterization and improvement of the reliability of hydrogel ionotronic devices.


 Please wait while we load your content...
Please wait while we load your content...