Interfacial behaviour of β-lactoglobulin aggregates at the oil–water interface studied using particle tracking and dilatational rheology†
Abstract
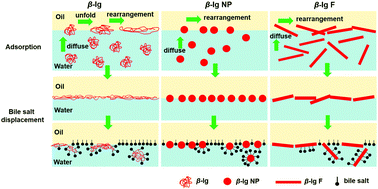
During processing, proteins are easily self-assembled into different aggregates, such as nanoparticles and fibrils. Protein aggregates exhibit a strong interfacial activity due to their morphologies and functional groups on the surface. Their interfacial structure and rheological properties at the oil–water interface have a significant effect on the stability and fat digestion of emulsions in food. In this study, β-lactoglobulin (β-lg) aggregates including β-lg nanoparticles (β-lg NP) and β-lg fibrils (β-lg F) were prepared in solution by controlling the heating temperature and pH, and their surface properties including the electric potential, hydrophobicity, and density of free thiol groups were characterized. The adsorption kinetics, interfacial rheology, and displacement by bile salts (BSs) of native β-lg and its aggregates at the oil (decane)/water interfaces were studied using particle tracking microrheology and dilatational rheology. From the movement of tracer particles at the interface, β-lg NP and β-lg F were found to adsorb faster than native β-lg, and they were found to form interfacial films with a marginally higher elasticity. During the process of protein adsorption, the films of β-lg and its aggregates are not uniform. In the process of protein displacement, β-lg NP has the strongest ability while native β-lg has the weakest ability to resist BS substitution, which is consistent with the results from in vitro digestion experiments. The present study reveals the microrheological behaviour of protein aggregates at the oil–water interface and demonstrates that β-lg thermal aggregates exhibit an excellent emulsification ability and can be used to control fat digestion. The study also illustrates the applicability of microrheological methods to the study of interfacial rheology and its complementarity with dilatational rheological methods.


 Please wait while we load your content...
Please wait while we load your content...