Surface tensiometry of phase separated protein and polymer droplets by the sessile drop method†
Abstract
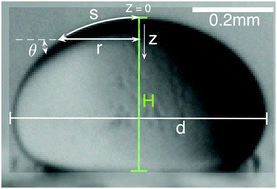
Phase separated macromolecules play essential roles in many biological and synthetic systems. Physical characterization of these systems can be challenging because of limited sample volumes, particularly for phase-separated proteins. Here, we demonstrate that a classic method for measuring the surface tension of liquid droplets, based on the analysis of the shape of a sessile droplet, can be effectively scaled down to measure the interfacial tension between a macromolecule-rich droplet phase and its co-existing macromolecule-poor continuous phase. The connection between droplet shape and surface tension relies on the density difference between the droplet and its surroundings. This can be determined with small sample volumes in the same setup by measuring the droplet sedimentation velocity. An interactive MATLAB script for extracting the capillary length from a droplet image is included in the ESI.


 Please wait while we load your content...
Please wait while we load your content...