Modification of gold nanoparticles with a hole-transferring cocatalyst: a new strategy for plasmonic water splitting under irradiation of visible light†
Abstract
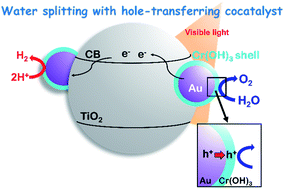
Plasmonic water splitting (H2O → H2 + 1/2O2) over a metal-loaded metal oxide under irradiation of visible light is still difficult, although conversion of organic compounds over plasmonic photocatalysts has become popular. Acceleration of oxygen (O2) production by water oxidation is the key for smooth water splitting. A chromium species was introduced to gold (Au)-loaded titanium(IV) oxide (Au/TiO2) by using the photodeposition method. The morphology, structure and electronic state of the chromium species and Au/TiO2 were analyzed by transmission electron microscopy, UV-vis spectroscopy, X-ray photoelectron spectroscopy and X-ray absorption spectroscopy. The results revealed that a very thin chromium hydroxide (Cr(OH)3) layer was formed on Au nanoparticles, which made the state of Au slightly electron-rich. The Cr(OH)3/Au/TiO2 plasmonic photocatalyst exhibited reaction rates larger than those of reactions over Cr(OH)3-free Au/TiO2 towards both water oxidation and water splitting under irradiation of visible light. Oxidative deposition of PbO2 revealed that plasmonic oxidation occurs on Cr(OH)3/Au and that Cr(OH)3 effectively works as the hole transfer cocatalyst. Based on the results, reaction mechanisms of plasmonic water oxidation and water splitting over Cr(OH)3/Au/TiO2 are proposed.


 Please wait while we load your content...
Please wait while we load your content...