Bimetallic cyclic redox couple in dimanganese copper oxide supported by nickel borate for boosted alkaline electrocatalytic oxygen evolution reaction†
Abstract
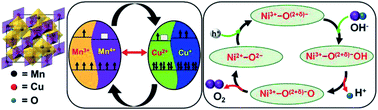
Flake-structured dimanganese copper oxide (Mn2CuO4), an unexplored binary transition metal oxide with mixed-valence states of Mn(III/IV) and Cu(II/I), is utilized as an electrocatalyst for oxygen evolution reaction (OER). An overpotential of 230 mV at 10 mA cm−2 and Tafel slope of 56 mV per decade is achieved with nickel borate (Ni–Bi) support, which is comparable to the benchmark RuO2 under similar experimental conditions. An average faradaic yield of ∼98% is achieved, indicating that any contribution from impurities is absent due to the use of alkaline medium. A turnover frequency increased by eight-fold is attained with the support of Ni–Bi by virtue of the Ni(II) ↔ Ni(III) redox couple. The mechanistic surface kinetics pathway at crossover potentials between the Mn2CuO4 and RuO2 systems is studied using electrochemical impedance. The efficiency of the modified catalyst can be attributed to the dual redox cycle of both the dimanganese copper oxide and nickel borate responsible for the charge transfer within the system. Further experiments were performed to check the effect of trace iron impurity in the standard reagent-grade chemicals.


 Please wait while we load your content...
Please wait while we load your content...