Mechanism of formaldehyde and formic acid formation on (101)-TiO2@Cu4 systems through CO2 hydrogenation†
Abstract
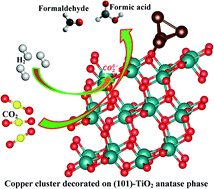
The decoration of a copper cluster on the anatase phase of a (101)-TiO2 surface to increase the reduction of CO2 has gained significant interest and potential to trigger sustainable solar-fuel-based economy. In the present work, we studied a heterogeneous surface for the reduction of CO2, which can produce various organic compounds such as formic acid, formaldehyde, methanol, ethanol, and methane. The density functional theory calculations were employed to study the formation of formaldehyde and methanol from CO2via hydrogenation by H2 on a Cu catalyst. The copper cluster is a unique catalyst for charge separation and conversion into important organic compounds. Theoretical investigations suggest that these organic compounds can be used as feedstock or be converted into solar fuel.


 Please wait while we load your content...
Please wait while we load your content...