Understanding the role of fluorination in the mechanistic nature of the water splitting process catalyzed by cobalt tris-(2-pyridylmethyl)amine complexes†
Abstract
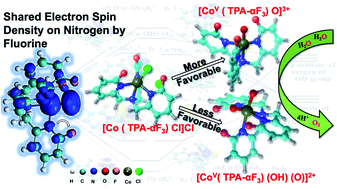
We report the reaction mechanism of the oxygen evolution reaction catalyzed by penta-coordinated [(CoV(TPA-αF3)(O))]3+ (TPA = tris-(2-pyridylmethyl)amine) and hexa-coordinated [CoV(TPA-αF3)(O)OH]2+ cobalt complexes for the formation of the oxygen–oxygen bond and the role of fluorination with the help of density functional theory. The nature of the orbitals involved in the formation of the oxygen–oxygen bond by these complexes is examined. The formation of the oxygen–oxygen bond occurs by the interaction of the dx2−y2* orbital of metal with the σ*-orbital of the hydroxide in the case of [(CoV(TPA-αF3)(O)OH]2+, while, in the case of [(CoV(TPA-αF3)(O))]3+, the dz2*-orbital accepted the electron from the hydroxide. The activation barrier for the oxygen–oxygen bond formation by the penta coordinated complex is lower than that of the hexa-coordinated complex. The release of oxygen through both the catalytic processes has nearly equal activation free energies. From the spin density analysis, we observe that the oxo-complexes are stabilized by sharing the spin density on the nitrogen atoms with fluorine atoms of the modified TPA ligand. Comparing the four-proton and four-electron process of the catalytic cycle with both catalytic species, it is found that the penta-coordinated complex ([(CoV(TPA-αF3)(O))]3+) requires a lower activation free energy barrier than the hexa-coordinated complex [CoV(TPA-αF3)(O)OH]2.


 Please wait while we load your content...
Please wait while we load your content...