1-Zirconacyclobuta-2,3-dienes: synthesis of organometallic analogs of elusive 1,2-cyclobutadiene, unprecedented intramolecular C–H activation, and reactivity studies†
Abstract
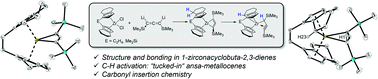
The structure, bonding, and reactivity of small, highly unsaturated ring systems is of fundamental interest for inorganic and organic chemistry. Four-membered metallacyclobuta-2,3-dienes, also referred to as metallacycloallenes, are among the most exotic examples for ring systems as these represent organometallic analogs of 1,2-cyclobutadiene, the smallest cyclic allene. Herein, the synthesis of the first examples of 1-zirconacyclobuta-2,3-dienes of the type [Cp′2Zr(Me3SiC3SiMe3)] (Cp′2 = rac-(ebthi), (ebthi = 1,2-ethylene-1,1′-bis(η5-tetrahydroindenyl)) (2a); rac-Me2Si(thi)2, thi = (η5-tetrahydroindenyl), (2b)) is presented. Both complexes undergo selective thermal C–H activation at the 7-position of the ansa-cyclopentadienyl ligand to produce a new type of “tucked-in” zirconocene system, 3a and 3b, that possesses a η3-propargyl/allenyl ligand. Both types of complexes react with carbonyl compounds, producing enynes in the case of 2a and 2b, as well as η1-allenyl complexes for 3a and 3b. Computational analysis of the structure and bonding of 2a and 3a reveals significant differences to a previously described related Ti complex. All complexes were fully characterised, including X-ray crystallography and experimental results were supported by DFT analysis.


 Please wait while we load your content...
Please wait while we load your content...