Synergetic stability enhancement with magnesium and calcium ion substitution for Ni/Mn-based P2-type sodium-ion battery cathodes†
Abstract
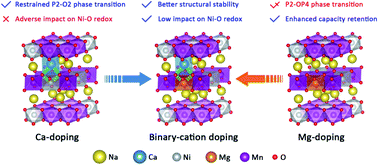
The conventional P2-type cathode material Na0.67Ni0.33Mn0.67O2 suffers from an irreversible P2–O2 phase transition and serious capacity fading during cycling. Here, we successfully carry out magnesium and calcium ion doping into the transition-metal layers (TM layers) and the alkali-metal layers (AM layers), respectively, of Na0.67Ni0.33Mn0.67O2. Both Mg and Ca doping can reduce O-type stacking in the high-voltage region, leading to enhanced cycling endurance, however, this is associated with a decrease in capacity. The results of density functional theory (DFT) studies reveal that the introduction of Mg2+ and Ca2+ make high-voltage reactions (oxygen redox and Ni4+/Ni3+ redox reactions) less accessible. Thanks to the synergetic effect of co-doping with Mg2+ and Ca2+ ions, the adverse effects on high-voltage reactions involving Ni–O bonding are limited, and the structural stability is further enhanced. The finally obtained P2-type Na0.62Ca0.025Ni0.28Mg0.05Mn0.67O2 exhibits a satisfactory initial energy density of 468.2 W h kg−1 and good capacity retention of 83% after 100 cycles at 50 mA g−1 within the voltage range of 2.2–4.35 V. This work deepens our understanding of the specific effects of Mg2+ and Ca2+ dopants and provides a stability-enhancing strategy utilizing abundant alkaline earth elements.


 Please wait while we load your content...
Please wait while we load your content...