Asymmetric synthesis of N–N axially chiral compounds via organocatalytic atroposelective N-acylation†‡
Abstract
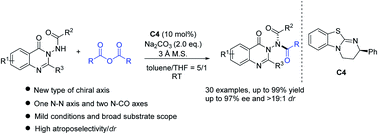
Compared with the well-developed C–C and C–N axial chirality, the asymmetric synthesis of N–N axial chirality remains elusive and challenging. Herein we report the first atroposelective N-acylation reaction of quinazolinone type benzamides with cinnamic anhydrides for the direct catalytic synthesis of optically active atropisomeric quinazolinone derivatives. This reaction features mild conditions and a broad substrate scope and produces N–N axially chiral compounds with high yields and very good enantioselectivities. Besides, the synthetic utility of the protocol was proved by a large scale reaction, transformation of the product and the utilization of the product as an acylation kinetic resolution reagent. Moreover, DFT calculations provide convincing evidence for the interpretation of stereoselection.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...