Metal-catalyzed B–H acylmethylation of pyridylcarboranes: access to carborane-fused indoliziniums and quinoliziniums†
Abstract
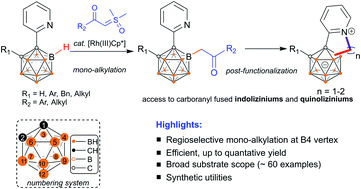
Metal-catalyzed mono-acylmethylation of pyridylcarboranes has been realized using α-carbonyl sulfoxonium ylides as a coupling partner. The reaction features high efficiency, excellent site-selectivity and good functional group tolerance. In the presence of pyridyl and enolizable acylmethyl groups, a post-coordination mode has been proposed and validated by in situ high resolution mass spectroscopy (HRMS) to rationalize the unique mono-substitution. Post-functionalization at the newly incorporated alkyl site provides additional utility of this method, including the construction of carborane-fused indoliziniums and quinoliziniums. We believe that these mono-alkylated carboranes, together with their post-functionalized derivatives, may find applications in luminescent materials and drug discovery in the near future.


 Please wait while we load your content...
Please wait while we load your content...