De novo design and synthesis of dipyridopurinone derivatives as visible-light photocatalysts in productive guanylation reactions†
Abstract
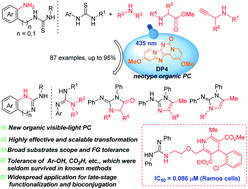
Described here is the de novo design and synthesis of a series of 6H-dipyrido[1,2-e:2′,1′-i]purin-6-ones (DPs) as a new class of visible-light photoredox catalysts (PCs). The synthesized DP1–5 showed their λAbs(max) values in 433–477 nm, excited state redox potentials 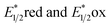 in 1.15–0.69 eV and −1.41 to −1.77 eV (vs. SCE), respectively. As a representative, DP4 enables the productive guanylation of various amines, including 1°, 2°, and 3°-alkyl primary amines, secondary amines, aryl and heteroaryl amines, amino-nitrile, amino acids and peptides as well as propynylamines and α-amino esters giving diversities in biologically important guanidines and cyclic guanidines. The photocatalytic efficacy of DP4 in the guanylation overmatched commonly used Ir and Ru polypyridyl complexes, and some organic PCs. Other salient merits of this method include broad substrate scope and functional group tolerance, gram-scale synthesis, and versatile late-stage derivatizations that led to a derivative 81 exhibiting 60-fold better anticancer activity against Ramos cells with the IC50 of 0.086 μM than that of clinical drug ibrutinib (5.1 μM).
in 1.15–0.69 eV and −1.41 to −1.77 eV (vs. SCE), respectively. As a representative, DP4 enables the productive guanylation of various amines, including 1°, 2°, and 3°-alkyl primary amines, secondary amines, aryl and heteroaryl amines, amino-nitrile, amino acids and peptides as well as propynylamines and α-amino esters giving diversities in biologically important guanidines and cyclic guanidines. The photocatalytic efficacy of DP4 in the guanylation overmatched commonly used Ir and Ru polypyridyl complexes, and some organic PCs. Other salient merits of this method include broad substrate scope and functional group tolerance, gram-scale synthesis, and versatile late-stage derivatizations that led to a derivative 81 exhibiting 60-fold better anticancer activity against Ramos cells with the IC50 of 0.086 μM than that of clinical drug ibrutinib (5.1 μM).


 Please wait while we load your content...
Please wait while we load your content...