Organic amine mediated cleavage of Caromatic–Cα bonds in lignin and its platform molecules†
Abstract
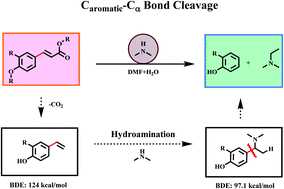
The activation and cleavage of C–C bonds remains a critical scientific issue in many organic reactions and is an unmet challenge due to their intrinsic inertness and ubiquity. Meanwhile, it is crucial for the valorization of lignin into high-value chemicals. Here, we proposed a novel strategy to enhance the Caromatic–Cα bond cleavage by pre-functionalization with amine sources, in which an active amine intermediate is first formed through Markovnikov hydroamination to reduce the dissociation energy of the Caromatic–Cα bond which is then cleaved to form target chemicals. More importantly, this strategy provides a method to achieve the maximum utilization of the aromatic nucleus and side chains in lignin or its platform molecules. Phenols and N,N-dimethylethylamine compounds with high yields were produced from herbaceous lignin or the p-coumaric acid monomer in the presence of industrially available dimethylamine (DMA).


 Please wait while we load your content...
Please wait while we load your content...