N–H⋯X interactions stabilize intra-residue C5 hydrogen bonded conformations in heterocyclic α-amino acid derivatives†
Abstract
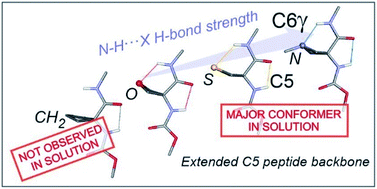
Nature makes extensive and elaborate use of hydrogen bonding to assemble and stabilize biomolecular structures. The shapes of peptides and proteins rely significantly on N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions, which are the linchpins of turns, sheets and helices. The C5 H-bond, in which a single residue provides both donor and acceptor, is generally considered too weak to force the backbone to adopt extended structures. Exploiting the synergy between gas phase (experimental and quantum chemistry) and solution spectroscopies to decipher IR spectroscopic data, this work demonstrates that the extended C5-based conformation in 4-membered ring heterocyclic α-amino acid derivatives is significantly stabilized by the formation of an N–H⋯X H-bond. In this synergic system the strength of the C5 interaction remains constant while the N–H⋯X H-bond strength, and thereby the support provided by it, varies with the heteroatom.
C interactions, which are the linchpins of turns, sheets and helices. The C5 H-bond, in which a single residue provides both donor and acceptor, is generally considered too weak to force the backbone to adopt extended structures. Exploiting the synergy between gas phase (experimental and quantum chemistry) and solution spectroscopies to decipher IR spectroscopic data, this work demonstrates that the extended C5-based conformation in 4-membered ring heterocyclic α-amino acid derivatives is significantly stabilized by the formation of an N–H⋯X H-bond. In this synergic system the strength of the C5 interaction remains constant while the N–H⋯X H-bond strength, and thereby the support provided by it, varies with the heteroatom.


 Please wait while we load your content...
Please wait while we load your content...