Artificial transmembrane signal transduction mediated by dynamic covalent chemistry†
Abstract
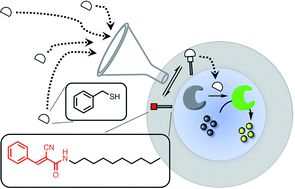
Reversible formation of covalent adducts between a thiol and a membrane-anchored Michael acceptor has been used to control the activation of a caged enzyme encapsulated inside vesicles. A peptide substrate and papain, caged as the mixed disulfide with methane thiol, were encapsulated inside vesicles, which contained Michael acceptors embedded in the lipid bilayer. In the absence of the Michael acceptor, addition of thiols to the external aqueous solution did not activate the enzyme to any significant extent. In the presence of the Michael acceptor, addition of benzyl thiol led to uncaging of the enzyme and hydrolysis of the peptide substrate to generate a fluorescence output signal. A charged thiol used as the input signal did not activate the enzyme. A Michael acceptor with a polar head group that cannot cross the lipid bilayer was just as effective at delivering benzyl thiol to the inner compartment of the vesicles as a non-polar Michael acceptor that can diffuse across the bilayer. The concentration dependence of the output signal suggests that the mechanism of signal transduction is based on increasing the local concentration of thiol present in the vesicles by the formation of Michael adducts. An interesting feature of this system is that enzyme activation is transient, which means that sequential addition of aliquots of thiol can be used to repeatedly generate an output signal.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...