Plasmonic O2 dissociation and spillover expedite selective oxidation of primary C–H bonds†
Abstract
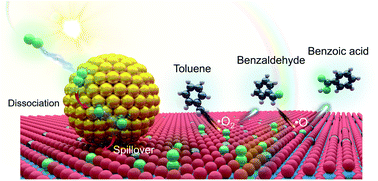
Manipulating O2 activation via nanosynthetic chemistry is critical in many oxidation reactions central to environmental remediation and chemical synthesis. Based on a carefully designed plasmonic Ru/TiO2−x catalyst, we first report a room-temperature O2 dissociation and spillover mechanism that expedites the “dream reaction” of selective primary C–H bond activation. Under visible light, surface plasmons excited in the negatively charged Ru nanoparticles decay into hot electrons, triggering spontaneous O2 dissociation to reactive atomic ˙O. Acceptor-like oxygen vacancies confined at the Ru–TiO2 interface free Ru from oxygen-poisoning by kinetically boosting the spillover of ˙O from Ru to TiO2. Evidenced by an exclusive isotopic O-transfer from 18O2 to oxygenated products, ˙O displays a synergistic action with native ˙O2− on TiO2 that oxidizes toluene and related alkyl aromatics to aromatic acids with extremely high selectivity. We believe the intelligent catalyst design for desirable O2 activation will contribute viable routes for synthesizing industrially important organic compounds.


 Please wait while we load your content...
Please wait while we load your content...