Ligand-redox assisted nickel catalysis toward stereoselective synthesis of (n+1)-membered cycloalkanes from 1,n-diols with methyl ketones†
Abstract
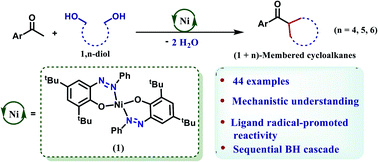
A well-defined, bench-stable nickel catalyst is presented here, that can facilitate double alkylation of a methyl ketone to realize a wide variety of cycloalkanes. The performance of the catalyst depends on the ligand redox process comprising an azo-hydrazo couple. The source of the bis electrophile in this double alkylation is a 1,n-diol, so that (n+1)-membered cycloalkanes can be furnished in a stereoselective manner. The reaction follows a cascade of dehydrogenation/hydrogenation reactions and adopts a borrowing hydrogen (BH) method. A thorough mechanistic analysis including the interception of key radical intermediates and DFT calculations supports the ligand radical-mediated dehydrogenation and hydrogenation reactions, which is quite rare in BH chemistry. In particular, this radical-promoted hydrogenation is distinctly different from conventional hydrogenations involving a metal hydride and complementary to the ubiquitous two-electron driven dehydrogenation/hydrogenation reactions.
- This article is part of the themed collection: Sustainable synthesis and catalysis – Chemical Science symposium collection


 Please wait while we load your content...
Please wait while we load your content...