Copper-catalyzed enantioselective carbonylation toward α-chiral secondary amides†
Abstract
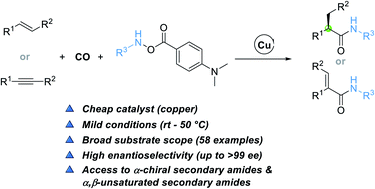
Secondary amides are omnipresent structural motifs in peptides, natural products, pharmaceuticals, and agrochemicals. The copper-catalyzed enantioselective hydroaminocarbonylation of alkenes described in this study provides a direct and practical approach for the construction of α-chiral secondary amides. An electrophilic amine transfer reagent possessing a 4-(dimethylamino)benzoate group was the key to the success. This method also features broad functional group tolerance and proceeds under very mild conditions, affording a set of α-chiral secondary amides in high yields (up to 96% yield) with unprecedented levels of enantioselectivity (up to >99% ee). α,β-Unsaturated secondary amides can also be produced though the method by using alkynes as the substrate.


 Please wait while we load your content...
Please wait while we load your content...