Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions†
Abstract
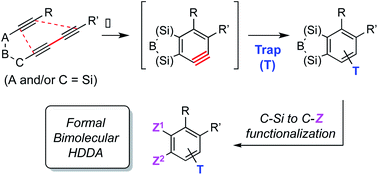
The 1,3-diyne and diynophile in hexadehydro-Diels–Alder (HDDA) reaction substrates are typically tethered by linker units that consist of C, O, N, and/or S atoms. We describe here a new class of polyynes based on silicon-containing tethers that can be disposed of and/or functionalized subsequent to the HDDA reaction. The cyclizations are efficient, and the resulting benzoxasiloles are amenable to protodesilylation, halogenation, oxygenation, and arylation reactions. The presence of the silicon atom can also override the innate mode of cyclization in some cases, an outcome attributable to a β-silyl effect on the structure of intermediate diradicals. Overall, this strategy equates formally to an otherwise unknown, bimolecular HDDA reaction and expands the versatility of this body of aryne chemistry.


 Please wait while we load your content...
Please wait while we load your content...