Catalytic asymmetric transformations of racemic α-borylmethyl-(E)-crotylboronate via kinetic resolution or enantioconvergent reaction pathways†
Abstract
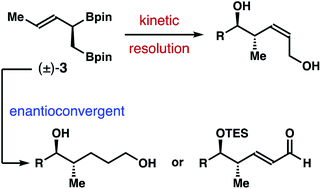
We report herein catalytic asymmetric transformations of racemic α-borylmethyl-(E)-crotylboronate. The Brønsted acid-catalyzed kinetic resolution–allylboration reaction sequence of the racemic reagent gave (Z)-δ-hydroxymethyl-anti-homoallylic alcohols with high Z-selectivities and enantioselectivities upon oxidative workup. In parallel, enantioconvergent pathways were utilized to synthesize chiral nonracemic 1,5-diols and α,β-unsaturated aldehydes with excellent optical purity.


 Please wait while we load your content...
Please wait while we load your content...