Unexpected inverse correlations and cooperativity in ion-pair phase transfer†
Abstract
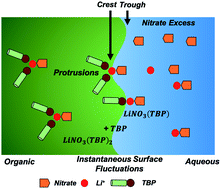
Liquid/liquid extraction is one of the most widely used separation and purification methods, where a forefront of research is the study of transport mechanisms for solute partitioning and the relationships that these have to solution structure at the phase boundary. To date, organized surface features that include protrusions, water-fingers, and molecular hinges have been reported. Many of these equilibrium studies have focused upon small-molecule transport – yet the extent to which the complexity of the solute, and the competition between different solutes, influence transport mechanisms have not been explored. Here we report molecular dynamics simulations that demonstrate that a metal salt (LiNO3) can be transported via a protrusion mechanism that is remarkably similar to that reported for H2O by tri-butyl phosphate (TBP), a process that involves dimeric assemblies. Yet the LiNO3 out-competes H2O for a bridging position between the extracting TBP dimer, which in-turn changes the preferred transport pathway of H2O. Examining the electrolyte concentration dependence on ion-pair transport unexpectedly reveals an inverse correlation with the extracting surfactant concentration. As [LiNO3] increases, surface adsorbed TBP becomes a limiting reactant in correlation with an increased negative surface charge induced by excess interfacial NO3−, however the rate of transport is enhanced. Within the highly dynamic interfacial environment, we hypothesize that this unique cooperative effect may be due to perturbed surface organization that either decreases the energy of formation of transporting protrusion motifs or makes it easier for these self-assembled species to disengage from the surface.


 Please wait while we load your content...
Please wait while we load your content...