Degradation and regeneration of Fe–Nx active sites for the oxygen reduction reaction: the role of surface oxidation, Fe demetallation and local carbon microporosity†
Abstract
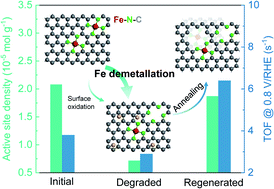
The severe degradation of Fe–N–C electrocatalysts during a long-term oxygen reduction reaction (ORR) has become a major obstacle for application in proton-exchange membrane fuel cells. Understanding the degradation mechanism and regeneration of aged Fe–N–C catalysts would be of particular interest for extending their service life. Herein, we show that the by-product hydrogen peroxide during the ORR not only results in the oxidation of the carbon surface but also causes the demetallation of Fe active sites. Quantitative analysis reveals that the Fe demetallation constitutes the main reason for catalyst degradation, while previously reported carbon surface oxidation plays a minor role. We further reveal that post thermal annealing of the aged catalysts can transform the oxygen functional groups on the carbon surface into micropores. These newly formed micropores not only help to increase the active-site density but also the intrinsic ORR activity of the neighbouring Fe–N4 sites, both contributing to complete activity recovery of aged Fe–N–C catalysts.


 Please wait while we load your content...
Please wait while we load your content...