A cationic thorium–organic framework with triple single-crystal-to-single-crystal transformation peculiarities for ultrasensitive anion recognition†
Abstract
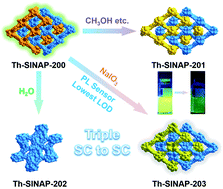
Single-crystal-to-single-crystal transformation of metal–organic frameworks has been met with great interest, as it allows for the creation of new materials in a stepwise manner and direct visualization of structural transitions when subjected to external stimuli. However, it remains a peculiarity among numerous metal–organic frameworks, particularly for the ones constructed from tetravalent metal cations. Herein, we present a cationic thorium–organic framework displaying unprecedented triple single-crystal-to-single-crystal transformations in organic solvents, water, and NaIO3 solution. Notably, both the interpenetration conversion and topological change driven by the SC–SC transformation have remained elusive for thorium–organic frameworks. Moreover, the single-crystal-to-single-crystal transition in NaIO3 solution can efficiently and selectively turn the ligand-based emission off, leading to the lowest limit of detection (0.107 μg kg−1) of iodate, one of the primary species of long-lived fission product 129I in aqueous medium, among all luminescent sensors.


 Please wait while we load your content...
Please wait while we load your content...