Discovery of SARS-CoV-2 Mpro peptide inhibitors from modelling substrate and ligand binding†
Abstract
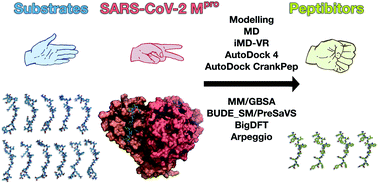
The main protease (Mpro) of SARS-CoV-2 is central to viral maturation and is a promising drug target, but little is known about structural aspects of how it binds to its 11 natural cleavage sites. We used biophysical and crystallographic data and an array of biomolecular simulation techniques, including automated docking, molecular dynamics (MD) and interactive MD in virtual reality, QM/MM, and linear-scaling DFT, to investigate the molecular features underlying recognition of the natural Mpro substrates. We extensively analysed the subsite interactions of modelled 11-residue cleavage site peptides, crystallographic ligands, and docked COVID Moonshot-designed covalent inhibitors. Our modelling studies reveal remarkable consistency in the hydrogen bonding patterns of the natural Mpro substrates, particularly on the N-terminal side of the scissile bond. They highlight the critical role of interactions beyond the immediate active site in recognition and catalysis, in particular plasticity at the S2 site. Building on our initial Mpro-substrate models, we used predictive saturation variation scanning (PreSaVS) to design peptides with improved affinity. Non-denaturing mass spectrometry and other biophysical analyses confirm these new and effective ‘peptibitors’ inhibit Mpro competitively. Our combined results provide new insights and highlight opportunities for the development of Mpro inhibitors as anti-COVID-19 drugs.
- This article is part of the themed collections: Most popular 2021 physical and theoretical chemistry articles, 2021 and 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...