‘Reverse biomimetic’ synthesis of l-arogenate and its stabilized analogues from l-tyrosine†
Abstract
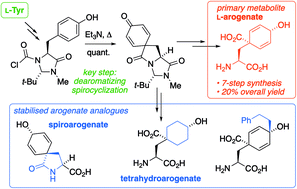
L-Arogenate (also known as L-pretyrosine) is a primary metabolite on a branch of the shikimate biosynthetic pathway to aromatic amino acids. It plays a key role in the synthesis of plant secondary metabolites including alkaloids and the phenylpropanoids that are the key to carbon fixation. Yet understanding the control of arogenate metabolism has been hampered by its extreme instability and the lack of a versatile synthetic route to arogenate and its analogues. We now report a practical synthesis of L-arogenate in seven steps from O-benzyl L-tyrosine methyl ester in an overall yield of 20%. The synthetic route also delivers the fungal metabolite spiroarogenate, as well as a range of stable saturated and substituted analogues of arogenate. The key step in the synthesis is a carboxylative dearomatization by intramolecular electrophilic capture of tyrosine's phenolic ring using an N-chloroformylimidazolidinone moiety, generating a versatile, functionalizable spirodienone intermediate.


 Please wait while we load your content...
Please wait while we load your content...