Trifluoromethyl substitution enhances photoinduced activity against breast cancer cells but reduces ligand exchange in Ru(ii) complex†
Abstract
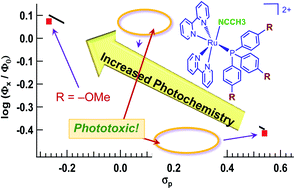
A series of five ruthenium complexes containing triphenyl phosphine groups known to enhance both cellular penetration and photoinduced ligand exchange, cis-[Ru(bpy)2(P(p-R-Ph)3)(CH3CN)]2+, where bpy = 2,2′-bipyridine and P(p-R-Ph)3 represent para-substituted triphenylphosphine ligands with R = –OCH3 (1), –CH3 (2) –H (3), –F (4), and –CF3 (5), were synthesized and characterized. The photolysis of 1–5 in water with visible light (λirr ≥ 395 nm) results in the substitution of the coordinated acetonitrile with a solvent molecule, generating the corresponding aqua complex as the single photoproduct. A 3-fold variation in quantum yield was measured with 400 nm irradiation, Φ400, where 1 is the most efficient with a Φ400 = 0.076(2), and 5 the least photoactive complex, with Φ400 = 0.026(2). This trend is unexpected based on the red-shifted metal-to-ligand charge transfer (MLCT) absorption of 1 as compared to that of 5, but can be correlated to the substituent Hammett para parameters and pKa values of the ancillary phosphine ligands. Complexes 1–5 are not toxic towards the triple negative breast cancer cell line MDA-MB-231 in the dark, but 3 and 5 are >4.2 and >19-fold more cytotoxic upon irradiation with blue light, respectively. A number of experiments point to apoptosis, and not to necrosis or necroptosis, as the mechanism of cell death by 5 upon irradiation. These findings provide a foundation for understanding the role of phosphine ligands on photoinduced ligand substitution and show the enhancement afforded by –CF3 groups on photochemotherapy, which will aid the future design of photocages for photochemotherapeutic drug delivery.


 Please wait while we load your content...
Please wait while we load your content...