“Concentration-in-Control” self-assembly concept at the liquid–solid interface challenged†
Abstract
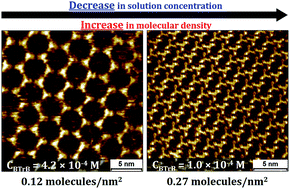
Self-assembled molecular networks (SAMNs) on surfaces evoke a lot of interest, both from a fundamental as well as application point of view. When formed at the liquid–solid interface, precise control over different polymorphs can be achieved by simply adjusting the concentration of molecular building blocks in solution. Significant influence of solute concentration on self-assembly behavior has been observed, whether the self-assembly behavior is controlled by either van der Waals forces or hydrogen bonding interactions. In both cases, high- and low-density supramolecular networks have been observed at high and low solute concentrations, respectively. In contrast to this “concentration-in-control” self-assembly concept here we report an atypical concentration dependent self-assembly behavior at a solution–solid interface. At the interface between heptanoic acid (HA) and highly oriented pyrolytic graphite (HOPG), we show, using scanning tunneling microscopy (STM), the formation of a low-density porous network at high solute concentrations, and a high-density compact network at low solute concentrations. This intriguing inverse concentration dependent self-assembly behavior has been attributed to the preaggregation of solute molecules in the heptanoic acid solution as revealed by UV-vis spectroscopy. The observed results have been correlated to the molecular density of self-assembled monolayers attained at the HA/HOPG interface.


 Please wait while we load your content...
Please wait while we load your content...