Expanding organofluorine chemical space: the design of chiral fluorinated isosteres enabled by I(i)/I(iii) catalysis†
Abstract
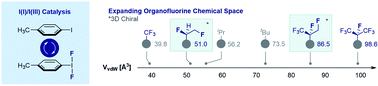
Short aliphatic groups are prevalent in bioactive small molecules and play an essential role in regulating physicochemistry and molecular recognition phenomena. Delineating their biological origins and significance have resulted in landmark developments in synthetic organic chemistry: Arigoni's venerable synthesis of the chiral methyl group is a personal favourite. Whilst radioisotopes allow the steric footprint of the native group to be preserved, this strategy was never intended for therapeutic chemotype development. In contrast, leveraging H → F bioisosterism provides scope to complement the chiral, radioactive bioisostere portfolio and to reach unexplored areas of chiral chemical space for small molecule drug discovery. Accelerated by advances in I(I)/I(III) catalysis, the current arsenal of achiral 2D and 3D drug discovery modules is rapidly expanding to include chiral units with unprecedented topologies and van der Waals volumes. This Perspective surveys key developments in the design and synthesis of short multivicinal fluoroalkanes under the auspices of main group catalysis paradigms.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...