Rapid one-pot iterative diselenide–selenoester ligation using a novel coumarin-based photolabile protecting group†
Abstract
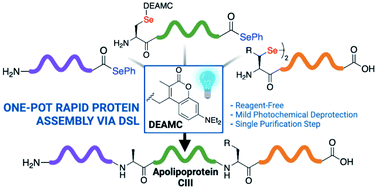
The development of an iterative one-pot peptide ligation strategy is described that capitalises on the rapid and efficient nature of the diselenide–selenoester ligation reaction, together with photodeselenisation chemistry. This ligation strategy hinged on the development of a novel photolabile protecting group for the side chain of selenocysteine, namely the 7-diethylamino-3-methyl coumarin (DEAMC) moiety. Deprotection of this DEAMC group can be effected in a mild, reagent-free manner using visible light (λ = 450 nm) without deleterious deselenisation of selenocysteine residues, thus enabling a subsequent ligation reaction without purification. The use of this DEAMC-protected selenocysteine in iterative DSL chemistry is highlighted through the efficient one-pot syntheses of 60- and 80-residue fragments of mucin-1 as well as apolipoprotein CIII in just 2–4 hours.


 Please wait while we load your content...
Please wait while we load your content...