IPr# – highly hindered, broadly applicable N-heterocyclic carbenes†
Abstract
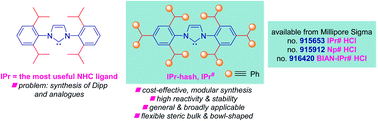
IPr (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) represents the most important NHC (NHC = N-heterocyclic carbene) ligand throughout the field of homogeneous catalysis. Herein, we report the synthesis, catalytic activity, and full structural and electronic characterization of novel, sterically-bulky, easily-accessible NHC ligands based on the hash peralkylation concept, including IPr#, Np# and BIAN-IPr#. The new ligands have been commercialized in collaboration with Millipore Sigma: IPr#HCl, 915653; Np#HCl; 915912; BIAN-IPr#HCl, 916420, enabling broad access of the academic and industrial researchers to new ligands for reaction optimization and screening. In particular, the synthesis of IPr# hinges upon cost-effective, modular alkylation of aniline, an industrial chemical that is available in bulk. The generality of this approach in ligand design is demonstrated through facile synthesis of BIAN-IPr# and Np#, two ligands that differ in steric properties and N-wingtip arrangement. The broad activity in various cross-coupling reactions in an array of N–C, O–C, C–Cl, C–Br, C–S and C–H bond cross-couplings is demonstrated. The evaluation of steric, electron-donating and π-accepting properties as well as coordination chemistry to Au(I), Rh(I) and Pd(II) is presented. Given the tremendous importance of NHC ligands in homogenous catalysis, we expect that this new class of NHCs will find rapid and widespread application.


 Please wait while we load your content...
Please wait while we load your content...