Ensemble effects in Cu/Au ultrasmall nanoparticles control the branching point for C1 selectivity during CO2 electroreduction†
Abstract
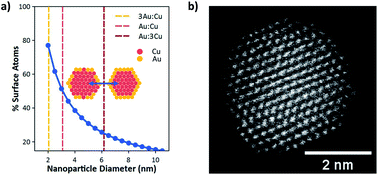
Bimetallic catalysts provide opportunities to overcome scaling laws governing selectivity of CO2 reduction (CO2R). Cu/Au nanoparticles show promise for CO2R, but Au surface segregation on particles with sizes ≥7 nm prevent investigation of surface atom ensembles. Here we employ ultrasmall (2 nm) Cu/Au nanoparticles as catalysts for CO2R. The high surface to volume ratio of ultrasmall particles inhibits formation of a Au shell, enabling the study of ensemble effects in Cu/Au nanoparticles with controllable composition and uniform size and shape. Electrokinetics show a nonmonotonic dependence of C1 selectivity between CO and HCOOH, with the 3Au:1Cu composition showing the highest HCOOH selectivity. Density functional theory identifies Cu2/Au(211) ensembles as unique in their ability to synthesize HCOOH by stabilizing CHOO* while preventing H2 evolution, making C1 product selectivity a sensitive function of Cu/Au surface ensemble distribution, consistent with experimental findings. These results yield important insights into C1 branching pathways and demonstrate how ultrasmall nanoparticles can circumvent traditional scaling laws to improve the selectivity of CO2R.


 Please wait while we load your content...
Please wait while we load your content...