“Click-switch” – one-step conversion of organic azides into photochromic diarylethenes for the generation of light-controlled systems†
Abstract
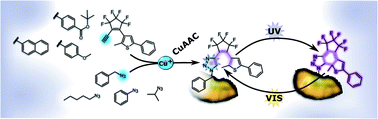
Diarylethenes (DAEs) are an established class of photochromic molecules, but their effective incorporation into pre-existing targets is synthetically difficult. Here we describe a new class of DAEs in which one of the aryl rings is a 1,2,3-triazole that is formed by “click” chemistry between an azide on the target and a matching alkyne–cyclopentene–thiophene component. This late-stage zero-length linking allows for tight integration of the DAE with the target, thereby increasing the chances for photomodulation of target functions. Nineteen different DAEs were synthesized and their properties investigated. All showed photochromism. Electron-withdrawing groups, and in particular −M-substituents at the triazole and/or thiophene moiety resulted in DAEs with high photo- and thermostability. Further, the chemical nature of the cyclopentene bridge had a strong influence on the behaviour upon UV light irradiation. Incorporation of perfluorinated cyclopentene led to compounds with high photo- and thermostability, but the reversible photochromic reaction was restricted to halogenated solvents. Compounds containing the perhydrogenated cyclopentene bridge, on the other hand, allowed the reversible photochromic reaction in a wide range of solvents, but had on average lower photo- and thermostabilities. The combination of the perhydrocyclopentene bridge and electron-withdrawing groups resulted in a DAE with improved photostability and no solvent restriction. Quantum chemical calculations helped to identify the photoproducts formed in halogenated as well as non-halogenated solvents. For two optimized DAE photoswitches, photostationary state composition and reaction quantum yields were determined. These data revealed efficient photochemical ring closure and opening. We envision applications of these new photochromic diarylethenes in photonics, nanotechnology, photobiology, photopharmacology and materials science.
- This article is part of the themed collection: Bioorthogonal and click chemistry: Celebrating the 2022 Nobel Prize in Chemistry


 Please wait while we load your content...
Please wait while we load your content...