Asymmetric synthesis of pharmaceutically relevant 1-aryl-2-heteroaryl- and 1,2-diheteroarylcyclopropane-1-carboxylates†
Abstract
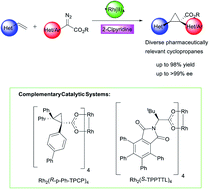
This study describes general methods for the enantioselective syntheses of pharmaceutically relevant 1-aryl-2-heteroaryl- and 1,2-diheteroarylcyclopropane-1-carboxylates through dirhodium tetracarboxylate-catalysed asymmetric cyclopropanation of vinyl heterocycles with aryl- or heteroaryldiazoacetates. The reactions are highly diastereoselective and high asymmetric induction could be achieved using either (R)-pantolactone as a chiral auxiliary or chiral dirhodium tetracarboxylate catalysts. For meta- or para-substituted aryl- or heteroaryldiazoacetates the optimum catalyst was Rh2(R-p-Ph-TPCP)4. In the case of ortho-substituted aryl- or heteroaryldiazoacetates, the optimum catalyst was Rh2(R-TPPTTL)4. For a highly enantioselective reaction with the ortho-substituted substrates, 2-chloropyridine was required as an additive in the presence of either 4 Å molecular sieves or 1,1,1,3,3,3-hexafluoroisopropanol (HFIP). Under the optimized conditions, the cyclopropanation could be conducted in the presence of a variety of heterocycles, such as pyridines, pyrazines, quinolines, indoles, oxadiazoles, thiophenes and pyrazoles.


 Please wait while we load your content...
Please wait while we load your content...