Do carbon nanotubes catalyse bromine/bromide redox chemistry?†
Abstract
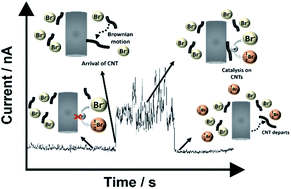
The redox chemistries of both the bromide oxidation and bromine reduction reactions are studied at single multi-walled carbon nanotubes (MWCNTs) as a function of their electrical potential allowing inference of the electron transfer kinetics of the Br2/Br− redox couple, widely used in batteries. The nanotubes are shown to be mildly catalytic compared to a glassy carbon surface but much less as inferred from conventional voltammetry on porous ensembles of MWCNTs where the mixed transport regime masks the true catalytic response.


 Please wait while we load your content...
Please wait while we load your content...